Rydberg Polarons
In school, we learn many things over and over again. One of these things are the states of matter. There are four basic states of matter: solid, liquid, plasma, and gas. Although, there has been a new state of matter that has been discovered: Rydberg matter. Many students, like Mathieu Senechal, are probably thinking, “I don’t even understand what you’re talking about.” In reality, matter can be defined into the different states by three two main principles: how fast the particles (atoms) move and how closely packed together they are. This can also be defined as the potential and kinetic energy possessed by the atoms within the substance and how closely packed together they are.
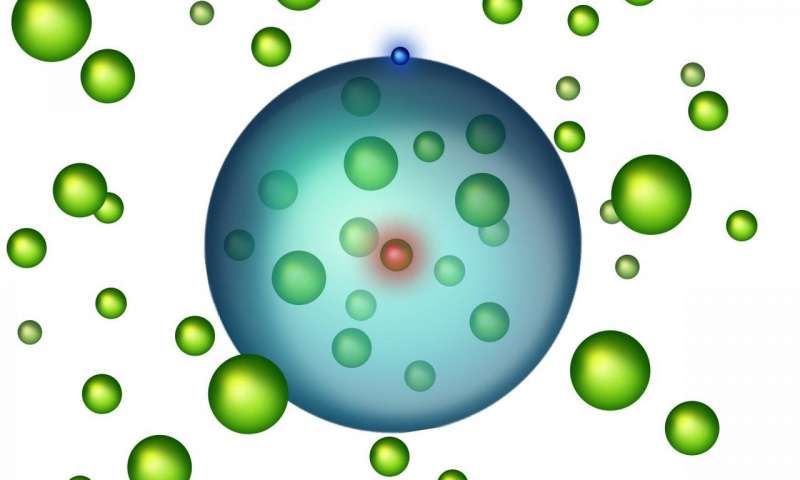
This state of matter can most easily be seen through illustrations because atoms are so small. To understand this new state, the parts of an atom must first be well understood. There are three basic parts that can make up any atom. The neutrons (neutral charge) and protons (positive charge) make up the nucleus of the atom. This is commonly the most stable part of any atom except inside of radioactive elements because when the nucleus splits apart it causes explosions and radioactivity. The electrons (negative charge) are in the electron cloud in rings around the nucleus. If the atom is large enough, then there is enough space inside the electron cloud for other smaller atoms to be able to fit. Most states of matter are naturally occurring and can transfer between the different states through the addition of energy. Contrastingly, this state has to be synthetically made and only lasts for a short while.
The official name for this state of matter is Rydberg Polarons. This is created by an excited strontium atom encompassing other strontium atoms. The first atom is excited by a laser; this means that the outer electron jumps up several rings for just part of a second which is long enough for other atoms to get inside the ring. This strontium atom that is excited can now fit other strontium atoms within its electron cloud.
Researcher Shuhei Yoshida from TU Wien is saying, “This is a highly unusual situation. Normally, we are dealing with charged nuclei, binding electrons around them. Here, we have an electron, binding neutral atoms.” These neutral atoms actually do disrupt the excited electron as it orbits its nucleus, but due to Quantum Physics, these electron can stay in orbit without altering its path. Luckily, neutral atoms carry no charge; therefore, they are of no hard to this fragile state of matter.
This new state of matter is also strange in the fact that it can only occur at extremely low temperatures. This makes it very difficult for researchers at TU Wien, a university in Austria, to be able to study Rydberg Polarons. While researchers are trying to keep the area at low enough temperatures, atoms give off heat making the effort almost useless. This now allows scientists to be able to study new ideas in ultracold environments.
While much of the research in ultracold environments is happening in other countries, including Austria, some of it is happening in our own backyard. Georgia Technological University just recently received a $900,000grant from the United States Air Force of Scientific Research to study atoms in ultracold temperatures as they approach absolute zero. (They would never reach absolute zero because then thermal activity would cease, causing reactions to stop taking place.) These extremely low temperatures will allow for atoms to function and interact with each other in new and interesting ways. One of these examples is the new state of matter, Rydberg Polarons. The works happening at Georgia Tech could also lead to a deeper understanding of how bonds form and what makes them stay together and break apart. These studies could then bring about new theories in chemistry which will then help explain why certain things happen at these ultracold temperatures.
This also affects the way wavelengths behave. When temperatures drop, the wavelength increases exponentially. Uzi Landman, a professor at the Georgia Tech department of physics gives an example by saying, “The wavelength of a particle, say a lithium atom, taken from room temperature to one nano-kelvin, grows by a factor of about 600,000, from about 0.04 nanometers at room temperature to 24,000 nanometers (24 microns) at the lower temperature – which is a very dramatic change.”
Also, the temperature drops cause electrons to be unable to bond with others; therefore, when the activation energy point cannot be reached, a wave effect begins. To try and see how atoms can bond in an ultracold environment, the scientists are going to try putting different types of atoms in the chamber in different amounts to observe their behaviors. They will begin with a small amount of atoms and work their way towards larger numbers.
Scientists can also control the position of atoms through lattices and then form synthetic solids in this way. This will allow for scientists and researchers to do studies on these synthetic solids that can not be created at room temperature. Unlike regular solids that are held together by chemical bonds, these solids will be held together by photon laser beams. This is different from anything ever done before.
Obviously, science is progressing at a much faster rate now more than ever and the world around us is constantly changing. Connor Chapman said, “I think it’s crazy that there’s so many different states of matter.” Everything goes hand in hand with one another, and the discovery of this new state of matter is leading to and can lead to so many new discoveries and explorations in chemistry, biology, physics, and other branches of science. While it may all seem like it does not really matter right now, small advances in science can actually be large ones in retrospect.
Sources:
http://www.newsweek.com/atoms-electrons-states-matter-physics-821884
http://www.news.gatech.edu/2015/10/28/entering-strange-world-ultra-cold-chemistry
https://phys.org/news/2018-02-creation-rydberg-polarons-bose-gas.html